Waiver Or Alteration Of Hipaa Chop Institutional Review
Information on this form is protected health information and subject to all privacy and security regulations under hipaa. page 1 of 2 instructional information for prior authorization. In order to be accepted by doctors and hospitals, a hipaa release authorization must have six core requirements. a valid authorization must contain certain required statements: requirement 1: a description that identifies the requested information in a “specific and meaningful fashion” (45 c. f. r. section 164. 508(c)(1)(i;.

Hipaa Authorization Essential Elements
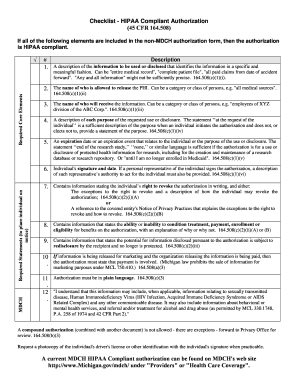
The core elements of a valid authorization include: a meaningful description of the information to be disclosed; the name of the individual or the name of the person authorized to make the requested disclosure. The authorization may not be combined with any other document such as a consent for treatment. 3 an authorization to use or disclose psychotherapy notes may not be combined with an authorization to disclose other forms of phi. 4; core elements. the authorization must contain the required “core elements” 5 -. inside a few short weeks the carter group required a newcustom programming features to track preventive maintenance essential to our continued success" business wire: webscheduler forms new division june 14, 2004 read the surgischeduler
In the medical-legal arena, hipaa sets forth standard “core requirements” that must be contained in a medical authorization to release patient information. this allows for health care providers and hospitals to continue accepting medical authorization forms from attorneys that contain those required elements without creating an overwhelming. Sample authorization language for research uses and disclosures of individually identifiable health information by a covered health care provider. authorization to use or disclose (release) health information that identifies you for a research study. required elements:. These elements include: a description of the specific information to be used or disclosed. the name or other specific identification of the person (s), or class of persons, authorized to make the requested use the name or other specific identification of any third parties (persons or classes of. Will the hipaa privacy rule hinder medical research by making doctors and others less willing and/or able to share with researchers information about individual patients?.
An authorization for research must be written in plain language and must contain all of the following elements: a specific and meaningful description of the information to be used or disclosed. the name or identification of the persons or class of persons authorized to make disclosures of phi and to use the phi for research-related purposes. The authorization must contain the required “core elements” 5 a description of the phi to be used or disclosed that identifies the phi in a specific and meaningful fashion. the name or specific identification of the person(s) or class of person(s) authorized to make the use or disclosure.
Hipaa Authorization Form What Is It And Why Do I Need One
Oct 12, 2020 · the prtf must submit the member’s individual plan of care and authorization form (dhs-7666-eng) (pdf) no later than 14 days after admission to the prtf. if the prtf does not submit the plan of care within the required 14 days, there is no guarantee bhd will review and authorize the plan of care before the days requested for authorization. (a) standard: authorizations for uses and disclosures (1) authorization required: general rule. except as otherwise permitted or required by this subchapter, a elements form authorization hipaa required covered entity may not use or disclose protected health information without an authorization that is valid under this section. when a covered entity obtains or receives a valid authorization for its use or disclosure of protected.
Hipaa authorization essential elements a description of the information to be used or disclosed that identifies the information in a specific and meaningful fashion. the name or other specific identification of the patient or class of persons, authorized to make the requested use or disclosure.
Mar 23, 2020 · we are following the same roi and hipaa guidelines for covid-19. as long as there is a valid authorization to release information from the patient or for continuity of care. we are offering patients the option to obtain their test results or any records via mychart so that they can access online. Checklist for incorporating elements for a hipaa authorization into a consent form. v. 4/12/21. it elements form authorization hipaa required is recommended that you use this tool to ensure that each element of the required hipaa authorization is included. this language may be used in a hybri d consent/authorization form or for a “stand -alone” authorization. The core elements of a valid authorization include: a meaningful description of the information to be disclosed the name of the individual or the name of the person authorized to make the requested disclosure the name or other identification of the recipient of the information a description of each. A covered entity must document and retain any signed authorization under this section as required by § 164. 530(j). (c) implementation specifications: core elements and requirements (1) core elements. a valid authorization under this section must contain at least the following elements:.
Hipaa authorization essential elements if the authorization is signed by a personal representative of the individual, a by the recipient and no longer be protected by the hipaa privacy regulation other requirements the authorization must be written in plain language. health and human services following are sure critical elements that one needs to consider: physical shields manages the get to and control over the data each one of those organizations or items that take after the hipaa compliance software must follow the guidelines and directions
Ahima Special Update Release Of Information Recommendations
45 cfr § 164. 508 uses and disclosures for which an.
What required statements must the hipaa authorization form elements form authorization hipaa required contain? in addition to the core elements, the hipaa authorization must contain statements adequate to place the individual on notice of all of the following: the individual ‘s right to revoke the authorization in writing. Core elements and requir ed statements that must be included in an authorization. an authorization is not valid unless it contains all the r equired elements and statements. an authorization form may also, but is not required to, include additional, optional elements so long as they are not inconsistent with the r equired.
The verbal consent/authorization must contain all of the required elements for a valid consent plus hipaa authorization. the investigator must explain how they will document that the subject gave verbal authorization for the use of phi. the investigator must make a compelling case that the research would not be practicable without the waiver. Answer: you may disclose the phi as long as the request is a valid authorization. the hipaa privacy rule sets forth six specific elements (including the patient’s signature) elements form authorization hipaa required and three required statements that must be included. if any one of the elements or statements is missing, the authorization is not valid. A covered entity must document and retain any signed authorization under this section as required by §164. 530(j). (c) implementation specifications: core elements and requirements. (1) core elements. a valid authorization under this section must contain at least the following elements:.
Hipaa authorization essential elements.
An authorization for research must be written in plain language and must contain all of the following elements: a specific and meaningful description of the information to be used or disclosed. the name or identification of the persons or class of persons authorized to make disclosures of phi and to. Apr 12, 2021 · healthcare data security is an important element of health insurance portability and accountability act rules. the hipaa security rule requires covered entities to assess data security controls by conducting a risk assessment, and implement a risk management program to address any vulnerabilities that are identified. hipaa-covered entities must also implement appropriate administrative. Once a company is in the process of having a patient sign a consent form, it is not much extra work to include the additional elements required for a hipaa-compliant authorization. this method makes it possible to obtain wider access to use of the data. An authorization is not valid unless it contains both the required core elements, and all of the required statements. this is the minimum information needed to ensure individuals are fully informed of their rights with respect to an authorization and to understand the consequences of authorizing the disclosure. the required statements.